The following guest post comes from Philip Stevens, Director of the Geneva Network, a research and advocacy organization working on international health, trade, and intellectual property issues. The original research note can be found here.
By Philip Stevens
 In the Trans-Pacific Partnership (TPP) negotiations, the U.S. and Japan have proposed that TPP partners increase their period of regulatory data protection (RDP) for biologic medicines to align with practice in other countries. These proposals have been strongly opposed by a number of academics, who claim that such a move would significantly increase public spending on medicines, thereby potentially limiting access.[1], [2]
In the Trans-Pacific Partnership (TPP) negotiations, the U.S. and Japan have proposed that TPP partners increase their period of regulatory data protection (RDP) for biologic medicines to align with practice in other countries. These proposals have been strongly opposed by a number of academics, who claim that such a move would significantly increase public spending on medicines, thereby potentially limiting access.[1], [2]
Past experiences in Canada and Japan, which lengthened their respective terms of RDP some years ago, however, indicate that these fears of budget increases are unlikely to materialise.
Canada and Japan increased RDP[i] substantially but did not experience increases in expenditures for medicines
Like several TPP countries, the governments of Canada and Japan have national health insurance systems, and cover most health care costs, including medicines. Unlike other TPP countries, Canada and Japan have in the past decade adopted substantially longer terms of RDP. Their experiences, captured in the data provided below, show that expenditures on medicines did not change appreciably from previous trends.
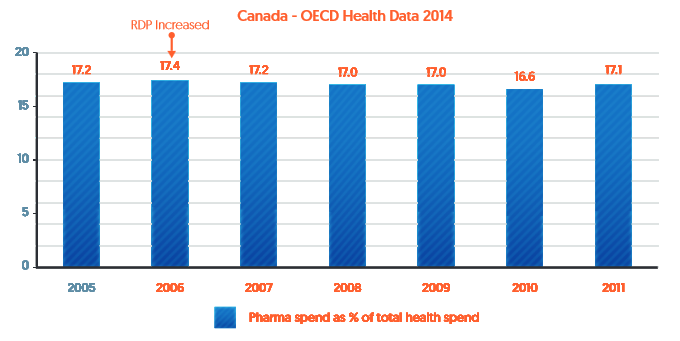
In 2006 Canada changed its regulations in a way that effectively increased their RDP term from 0 years to 8 years.[ii] As shown in Figure 1 (based on 2014 OECD data[iii]), pharmaceutical spending as a percentage of total health spending has actually decreased since then.
Figure 1: Pharmaceutical expenditure as a percentage of Canada’s healthcare expenditure (2005-2011)
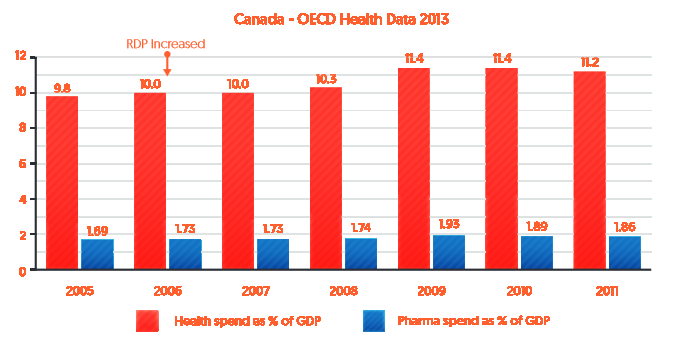
As indicated in Figure 2 below, over the same period (2005-2011) pharmaceutical expenditure as a percentage of GDP (blue bars) remained relatively stable after RDP was increased in Canada in 2006, whereas overall health spending as a percentage of GDP in Canada has gradually increased (red bars).
Figure 2: Health and pharmaceutical expenditure as a percentage of Canada’s GDP (2005-2011)
Similarly, Japan increased data protection in 2007 from 6 to 8 years (effectively 9 years).[iv] As indicated by Figure 3, fluctuations in expenditures after that time have been in line with growth in health care spending as a percentage of GDP. In fact, in 2010 pharmaceutical spending decreased in a year where health care spending increased.
Figure 3: Pharmaceutical expenditure as a percentage of Japan’s health care expenditure (2005-2010)
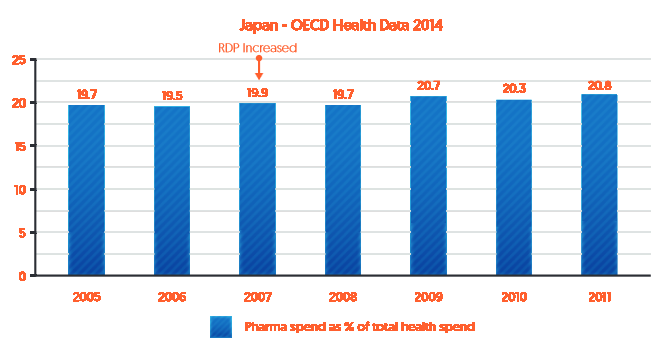
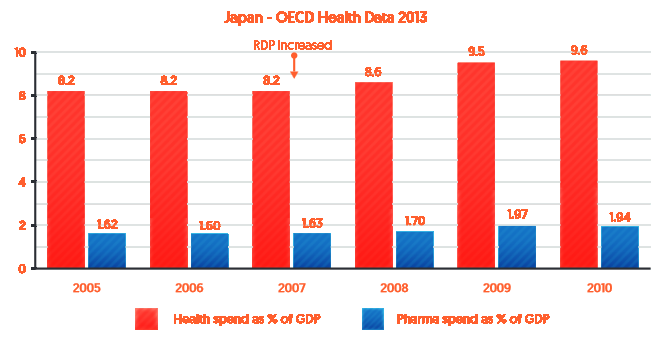
Figure 4 shows that the gradual increases in pharmaceutical expenditure as a percentage of GDP in Japan between 2005 and 2010 (blue bars) was in line with the overall increase in health spending as a percentage of GDP in Japan over the same period (red bars).
Figure 4: Health and pharmaceutical expenditure as a percentage of Japan’s GDP (2005-2010)
Conclusion
The past experiences of Canada and Japan described above indicate that increases in RDP terms do not result in meaningful increases in health care expenditures or expenditures on medicines relative to overall health care spending. There could be many explanations for this result, ranging from changes in procurement policies, to increases in the number of medicines whose patent terms have expired. The evidence presented above, however, suggests that those concerned about access to medicines and the financial sustainability of public healthcare systems should focus their attention on policies other than Regulatory Data Protection for medicines.
[1] Moir et al, (2014) “Proposals for extending data protection for biologics in the TPPA: Potential consequences for Australia”, Submission to the Department of Foreign Affairs and Trade, available at http://dfat.gov.au/trade/agreements/tpp/submissions/Documents/tpp_sub_gleeson_lopert_moir.pdf
[2] Gleeson, D, Lopert, R, and Reid, P, (2013), “How the Trans Pacific Partnership Agreement could undermine PHARMAC and threaten access to affordable medicines and health equity in New Zealand”, Health Policy, 116:2-3
[i] Japan has a “post marketing surveillance system” which we consider a surrogate for RDP and use the term RDP in this paper to include Japan’s approach.
[ii] Canada’s 5-year data protection term was made ineffective by a 1998 Federal Court interpretation of regulations. Bayer Inc. v. Canada (Attorney General), 84 C.P.R. (3d) 129, aff’d 87 C.P.R. (3d) 293, leave to appeal to SCC refused, [1999] S.C.C.A. No. 386. The Federal Court held that RDP protection in Canada was not triggered if a generic applicant could demonstrate bioequivalence without requiring the Health Minister to consult the data submitted by the innovative company. Because that was a common occurrence, RDP rarely applied under the pre-2006 regulations.
[iii] 2013/14 OECD data on Canada and Japan is found at: http://www.oecd-ilibrary.org/social-issues-migration-health/health-key-tables-from-oecd_20758480;jsessionid=k26q30wbgljb.x-oecd-live-02.
[iv] Japan’s system prevents filing applications for follow-on approval for eight years after the innovator’s approval. An additional year after that is required for the regulatory approval process to conclude.